Scratch Beneath the Surface: ABSSSI - Pharmacy Times
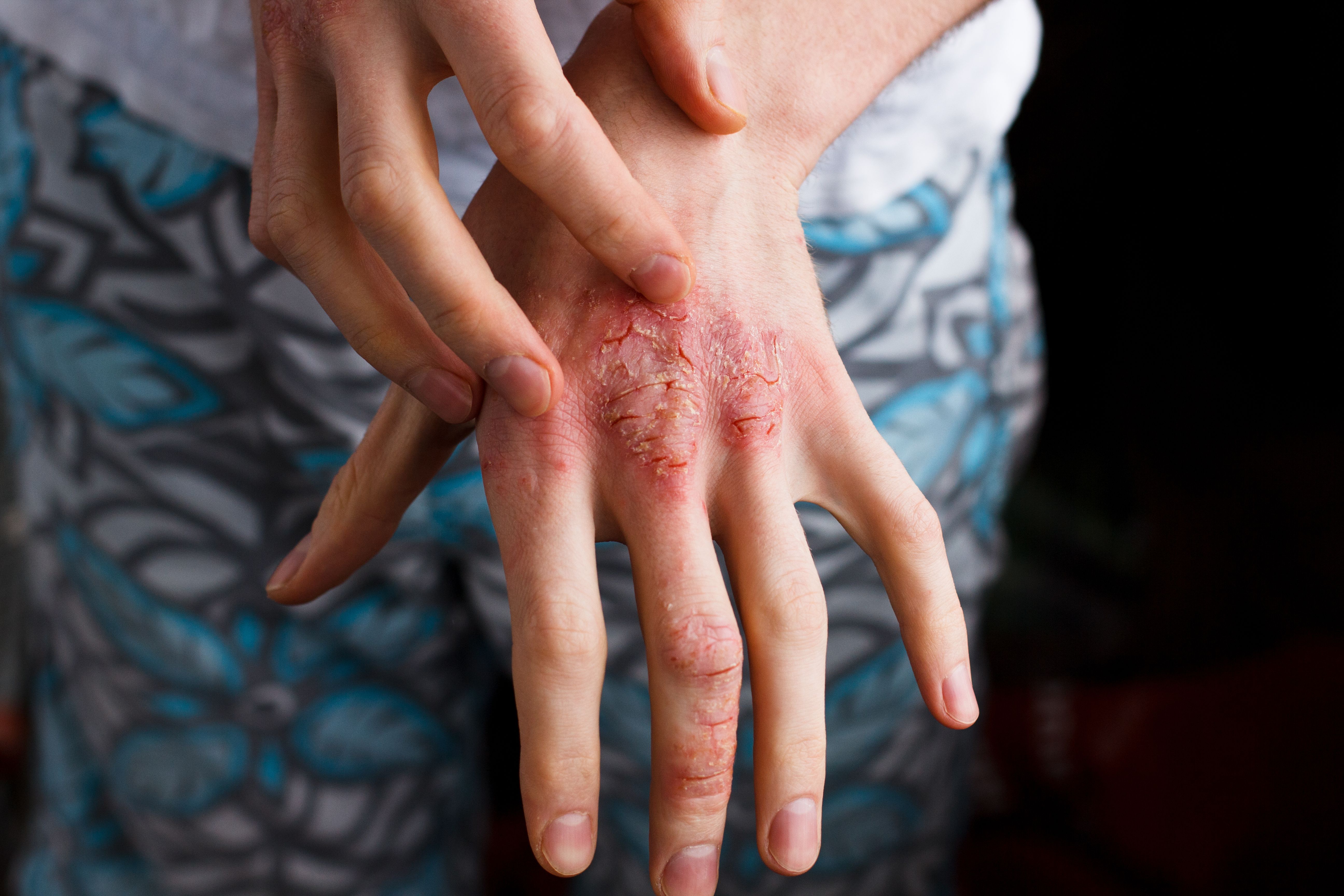
Consider key elements of acute bacterial skin and skin structure infection to help patients manage this broad range of diseases.
The terms skin and skin structure infection and skin and soft tissue infection encompass a broad spectrum of diseases affecting skin and underlying subcutaneous tissue, fascia, or muscle that can range in severity from mild to life threatening.1,2
Clinical trials using this terminology leave some ambiguity in interpretation because of the use of heterogeneous, broad inclusion criteria attributed to a lack of standardized nomenclature and monitoring parameters.1,3 To provide consistency in the identification of infections for which a treatment effect can be reliably estimated, in October 2013 the FDA released a guidance document that introduced the term acute bacterial skin and skin structure infections (ABSSSI) and defined quantifiable efficacy end points.1,3,4
DIAGNOSIS
ABSSSI include cellulitis/erysipelas, wound infections, and major cutaneous abscesses with a lesion surface area of equal to or greater than 75 cm2, measured by the area of edema, induration, or redness.4 Excluded from the definition are some skin and skin structure infections of very mild severity, very high severity, or those appearing from specific causes or in specific hosts. Such conditions include animal or human bites, burn wounds, chronic wound or diabetic food infections, ecthyma gangrenosum, impetigo, minor cutaneous abscesses, myonecrosis, and necrotizing fasciitis.4
Suboptimal treatment of ABSSSI, resulting from failure to recognize risk factors, misclassification of severity, misdiagnosis, or using the wrong duration or type of antibiotics, may lead to infection recurrence and/or treatment failure.5 This review summarizes the treatment of ABSSSI by focusing on typical pathogens expected, based on severity classification and patient risk factors, and provides associated recommendations for treatment.
COMMON BACTERIAL PATHOGENS
Pathogens that should be suspected in various ABSSSI depend on the severity classification and patient risk factors. Abscesses and cellulitis/erysipelas are typically caused by gram-positive bacteria. However, other pathogens can be encountered depending on the location of infection and other risk factors.5-7 The presence of purulence typically indicates a high probability that Staphylococcus aureus, as opposed to a streptococcal spp, may be involved. In wound infections, the anatomical location of the wound plays a significant role in determining the microbiologic differential.5 For example, gram-negative organisms are typically seen in wound infections of the lower or perianal abdominal area, whereas methicillin-resistant S aureus (MRSA) and methicillin-susceptible S aureus (MSSA) are more predominant in wound infections following surgery of the extremities, head, neck, or trunk.5,6
EMPIRIC ANTIMICROBIAL THERAPY
The guideline-recommended empiric antibiotic regimens differ for nonpurulent and purulent infections, because of known differences in causative pathogens. For purulent infections, empiric oral antibiotic regimens for mild or moderate disease include oral agents with activity against S aureus, including MRSA and MSSA, such as doxycycline or trimethoprim-sulfamethoxazole.1 For patients with moderate or severe purulent infections requiring inpatient admission, intravenous (IV) agents include ceftaroline, daptomycin, linezolid, or vancomycin, with vancomycin representing the typical first-line agent.1 As in all infectious syndromes, special care should be given to the appropriate stewardship of antimicrobial agents when making therapy selections.
Empiric antibiotic regimens for nonpurulent cellulitis and erysipelas should primarily target streptococcal spp given the predominance of this organism in the syndrome. For outpatient management of mild or moderate infections, optimal agents include either oral first-generation cephalosporins (ie, cefadroxil or cephalexin) or a penicillin (ie, amoxicillin or penicillin). Clindamycin can be used for patients with known, severe β-lactam allergy.6 For inpatient management of mild or moderate infections, the optimal first-line IV option is a first-generation cephalosporin, such as cefazolin, although such other agents as ceftriaxone, nafcillin, or penicillin also provide an appropriate empiric spectrum of activity. Clindamycin or vancomycin can be used in patients unable to tolerate β-lactams.
Patients with nonpurulent disease who have a history of MRSA infections should be considered for empiric treatment with vancomycin or another agent that will cover MRSA.6
As previously indicated, treatment of wound infections varies based on the anatomical location of the wound and expected flora at the wound site. In general, oral antimicrobial regimens should include antistreptococcal agents, such as cephalexin, and anti-MRSA agents, such as trimethoprim-sulfamethoxazole. IV vancomycin provides appropriate coverage of gram-positive organisms for patients admitted to the hospital.7 Infected wounds of the abdominal, perianal, or urogenital regions should include empiric coverage for enteric anaerobes and gram-negative pathogens.8 Empiric oral regimens include amoxicillin-clavulanate or cefdinir and metronidazole. A preferred empiric IV regimen for abdominal, perineal, or urogenital wound infection consists of ceftriaxone and metronidazole.1,8
DURATION OF THERAPY
In attempts to reduce the risk for unintended consequences of antimicrobial use, significant focus has been applied to reducing the duration of antibiotic treatment for many infections, including ABSSSI. Studies continue to emerge showing that shorter courses of antibiotics for cellulitis are just as efficacious as longer durations.9-11 In 1 such study of patients receiving antibiotic therapy for uncomplicated cellulitis, the results showed no significant differences in the clinical outcomes or treatment failure in those who received 5 days of therapy compared with patients receiving 10 days.12 As such, the most recent guidelines for ABSSSI recommend a duration of 5 days of antibiotic therapy. However, for those who demonstrate delayed clinical improvement (ie, delayed resolution of fever, recession of erythema, or vital sign stability), it may be necessary to extend the antimicrobial duration.6
CONCLUSION
ABSSSI are commonly encountered in clinical practice. The broad scope of patient risk factors, severity of illness, and syndromes can be intimidating factors in determining suspected microbiology, empiric antimicrobial selection, and therapy duration. Key elements involved in these facets of care include the absence or presence of purulence, anatomical location, need for inpatient care or IV antimicrobials, and severity of infection. Careful consideration of these key factors will guide clinicians in their care of patients presenting with ABSSSI (see Table6-8,13,14).
ABOUT THE AUTHORS
Sasha Premraj, PharmD, is an inpatient antimicrobial stewardship pharmacist at Ascension Sacred Heart Hospital in Pensacola, Florida.
Ryan Stevens, PharmD, BCPS, is an antimicrobial stewardship/outpatient antimicrobial therapy pharmacist at Mayo Clinic in Rochester, Minnesota.
Kellyn Engstrom, PharmD, MPH, is a PGY-2 emergency medicine pharmacy resident at Mayo Clinic.
REFERENCES
1. Pollack CV Jr, Amin A, Ford WT Jr, et al. Acute bacterial skin and skin structure infections (ABSSSI): practice guidelines for management and care transitions in the emergency department and hospital. J Emerg Med. 2015;48(4):508-519. doi:10.1016/j.jemermed.2014.12.001
2. Tiwari AK, Lal R. Study to evaluate the role of severity stratification of skin and soft tissue infections (SSTIs) in formulating treatment strategies and predicting poor prognostic factors. Int J Surg. 2014;12(2):125-133. doi:10.1016/j.ijsu.2013.11.014
3. Russo A, Concia E, Cristini F, et al. Current and future trends in antibiotic therapy of acute bacterial skin and skin-structure infections. Clin Microbiol Infect.2016;22(suppl 2):S27-36.doi:10.1016/S1198-743X(16)30095-7
4. Acute bacterial skin and skin structure infections: developing drugs for treatment. FDA. October 2013. Accessed June 18, 2021. https://www.fda.gov/files/drugs/published/Acute-Bacterial-Skin-and-Skin-Structure-Infections---Developing-Drugs-for-Treatment.pdf
5. Pulido-Cejudo A, Guzmán-Gutierrez M, Jalife-Montaño A, et al. Management of acute bacterial skin and skin structure infections with a focus on patients at high risk of treatment failure. Ther Adv Infect Dis.2017;4(5):143-161. doi:10.1177/2049936117723228
6. Stevens DL, Bisno AL, Chambers HF, et al; Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10-e52. doi:10.1093/cid/ciu444
7. Sartelli M, Guirao X, Hardcastle TC, et al. 2018 WSES/SIS-E consensus conference: recommendations for the management of skin and soft-tissue infections. World J Emerg Surg. 2018;13:58. doi:10.1186/s13017-018-0219-9
8. Mazuski JE, Tessier JM, May AK, et al. The Surgical Infection Society revised guidelines on the management of intra-abdominal infection. Surg Infect (Larchmt). 2017;18(1):1-76. doi:10.1089/sur.2016.261
9. Spellberg B. The new antibiotic mantra—"shorter is better." JAMA Intern Med. 2016;176(9):1254-1255. doi:10.1001/jamainternmed.2016.3646
10. Prokocimer P, De Anda C, Fang E, Mehra P, Das A. Tedizolid phosphate vs linezolid for treatment of acute bacterial skin and skin-structure infections: the ESTABLISH-1 randomized trial. JAMA. 2013;309(6):559-569. doi:10.1001/jama.2013.241
11. Moran GJ, Fang E, Corey GR, Das AF, De Anda C, Prokocimer P. Tedizolid for 6 days versus linezolid for 10 days for acute bacterial skin and skin-structure infections (ESTABLISH-2): a randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis. 2014;14(8):696-705. doi:10.1016/S1473-3099(14)70737-6
12. Hepburn MJ, Dooley DP, Skidmore PJ, Ellis MW, Starnes WF, Hasewinkle WC. Comparison of short course (5 days) and standard (10 days) treatment for uncomplicated cellulitis. Arch Intern Med. 2004;164(15):1669-1674. doi:10.1001/archinte.164.15.1669
13. Kannangara DW, Pandya D. Infections in injection drug users: the significance of oral bacteria and a comparison with bacteria originating from skin and environmental sources. Science Repository. January 22, 2020. Accessed June 18, 2021. https://www.researchgate.net/publication/338836628_Infections_in_Injection_Drug_Users_The_Significance_of_Oral_Bacteria_and_a_Comparison_with_Bacteria_Originating_from_Skin_and_Environmental_Sources
14. Jenkins TC, Knepper BC, Moore SJ, et al. Microbiology and initial antibiotic therapy for injection drug users and non-injection drug users with cutaneous abscesses in the era of community-associated methicillin-resistant Staphylococcus aureus. Acad Emerg Med. 2015;22(8):993-997. doi:10.1111/acem.12727

Comments
Post a Comment